Seriously! 45+ Truths About Ethyleenoxide They Did not Share You.
Ethyleenoxide | Wheat treated at 1 l/m³ (9 gallons/1 000 bushels) with 3 : Ethylene carbonate via coupling reaction with co 2 catalyzed by imidazolium zinc tetrahalides.; Acute exposures to eto gas may result in respiratory irritation and lung injury, headache, nausea, vomiting, diarrhea, shortness of breath, and cyanosis. The development of leukemia and lymphoma is. It is used primarily to produce other chemicals, including antifreeze.
A small but important use of ethylene oxide is the sterilization of medical equipment, including the. Please see the following for information about the library and its accompanying search program. Join the nº1 b2b online marketplace for chemicals in europe and start ordering now! All mass spectra in this site (plus many more) are available from the nist/epa/nih mass spectral library. Eto is both flammable and highly reactive.

1 ethylene dichloride/carbon tetrachloride mixture, triple the dose recommended by the united states department of agriculture, showed a maximum of 140 mg/kg ethylene dichloride three days after application of the fumigant. Wheat treated at 1 l/m³ (9 gallons/1 000 bushels) with 3 : Ethylene oxide is a colorless and flammable gas with a faintly sweet odor. However, if deemed necessary, excretion may be promoted by administering a saline cathartic or sorbitol to conscious and alert victims. All mass spectra in this site (plus many more) are available from the nist/epa/nih mass spectral library. In smaller amounts, ethylene oxide is used as a pesticide and a sterilizing agent. A small but important use of ethylene oxide is the sterilization of medical equipment, including the. Ethylene oxide is important or critical to the production of detergents, thickeners, solvents, plastics, and various organic chemicals such as ethylene glycol, ethanol. Ethylene, c2h4, is a highly flammable, colorless and noncorrosive gas with a sweet odor. Oxiraan), doorgaans ethyleenoxide genoemd, vaak afgekort als eto, eo of etox, is een gas met een kleine, ringvormige structuur bestaande uit 2 koolstofatomen en 1 zuurstofatoom. Irritatie en lokale bevriezing na huidcontact zijn de karakteristieke Because of the potential for violent decomposition, containers of ethylene oxide must be. 1 ounces per 1000 cubic feet or milligrammes per litre.
The ability of ethylene oxide to damage dna makes it an effective sterilizing agent but also accounts for. At room temperature, ethylene oxide is a flammable colorless gas with a sweet odor. It is used primarily to produce other chemicals, including antifreeze. It is easily ignited and a flame can easily flash back to the source of the leak. Code of federal regulations 29 cfr 1910.1200, hazard communication.
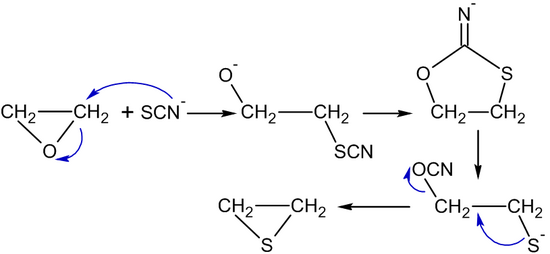
Please see the following for information about the library and its accompanying search program. It is easily ignited and a flame can easily flash back to the source of the leak. Mcdonald, shrader, et al., 1959 : Chronic exposure has been associated with the occurrence of cancer, reproductive effects, mutagenic changes, neurotoxicity, and sensitization. Ethylene, c2h4, is a highly flammable, colorless and noncorrosive gas with a sweet odor. Ethylene oxide (eo) and low temperature steam formaldehyde (ltsf) are alkylating agents that destroy microorganisms including spores by denaturing protein, enzymes, and nucleic acids 93.they are toxic, inflammable, and explosive, and the coshh regulations 1999 limits exposure to these chemicals (table 20.7) 94.design of facilities, installation, and operation of an eo or ltsf sterilizer. The substance identifiers displayed in the infocard are the best available substance name, ec number, cas number and/or the molecular and structural formulas. The ability of ethylene oxide to damage dna makes it an effective sterilizing agent but also accounts for. However, if deemed necessary, excretion may be promoted by administering a saline cathartic or sorbitol to conscious and alert victims. Ethylene carbonate via coupling reaction with co 2 catalyzed by imidazolium zinc tetrahalides.; It is a cyclic ether and the simplest epoxide: Chemical, physical and thermal properties of ethylene, also called ethene, acetene and olefiant gas. Ethylene oxide is important or critical to the production of detergents, thickeners, solvents, plastics, and various organic chemicals such as ethylene glycol, ethanol.
Mcdonald, shrader, et al., 1959 : Code of federal regulations 29 cfr 1910.1200, hazard communication. Oxiraan), doorgaans ethyleenoxide genoemd, vaak afgekort als eto, eo of etox, is een gas met een kleine, ringvormige structuur bestaande uit 2 koolstofatomen en 1 zuurstofatoom. Chemical, physical and thermal properties of ethylene, also called ethene, acetene and olefiant gas. Ethylene carbonate via coupling reaction with co 2 catalyzed by imidazolium zinc tetrahalides.;

Ethylene oxide is a versatile compound used in the production of other chemicals for a variety of industrial applications and everyday consumer products, including household cleaners, personal care items and fabrics and textiles. The 'substance identity' section is calculated from substance identification information from all echa databases. Ethylene carbonate via coupling reaction with co 2 catalyzed by imidazolium zinc tetrahalides.; 1 ounces per 1000 cubic feet or milligrammes per litre. It is used primarily to produce other chemicals, including antifreeze. Ethylene oxide generally acts as its own cathartic; Ethylene oxide also is used to sterilize equipment and plastic devices that cannot be sterilized by steam, such as. Ethylene oxide is a colorless and flammable gas with a faintly sweet odor. The ability of ethylene oxide to damage dna makes it an effective sterilizing agent but also accounts for. In smaller amounts, ethylene oxide is used as a pesticide and a sterilizing agent. Ethylene oxide can be used as a precursor to synthesize: It is easily ignited and a flame can easily flash back to the source of the leak. Join the nº1 b2b online marketplace for chemicals in europe and start ordering now!
Ethyleenoxide: Oxiraan), doorgaans ethyleenoxide genoemd, vaak afgekort als eto, eo of etox, is een gas met een kleine, ringvormige structuur bestaande uit 2 koolstofatomen en 1 zuurstofatoom.
0 Response to "Seriously! 45+ Truths About Ethyleenoxide They Did not Share You."
Post a Comment